Human factors are a vital part of medical device development
When we think about medical devices, we want them to be safe, effective, and perform as intended. During the development of medical devices, manufacturers must carry out extensive risk management and consider all possible risks. Safety during use should become the number 1 priority for all manufacturers.
An important aspect of medical device safety is the human factor. Human factors, or usability engineering, focuses on the interactions between people (users) and devices. Human factors are a key part of medical device development, and it is best if these are addressed early on in the development process. The main question the manufacturer has to ask is: will the average user understand how to operate the device correctly? As manufacturers, we would love to hope that all medical devices are safe and easy to use, but a Johns Hopkins study has found that an estimated 15% of medical error deaths are related to medical device user interface difficulties or errors [1].
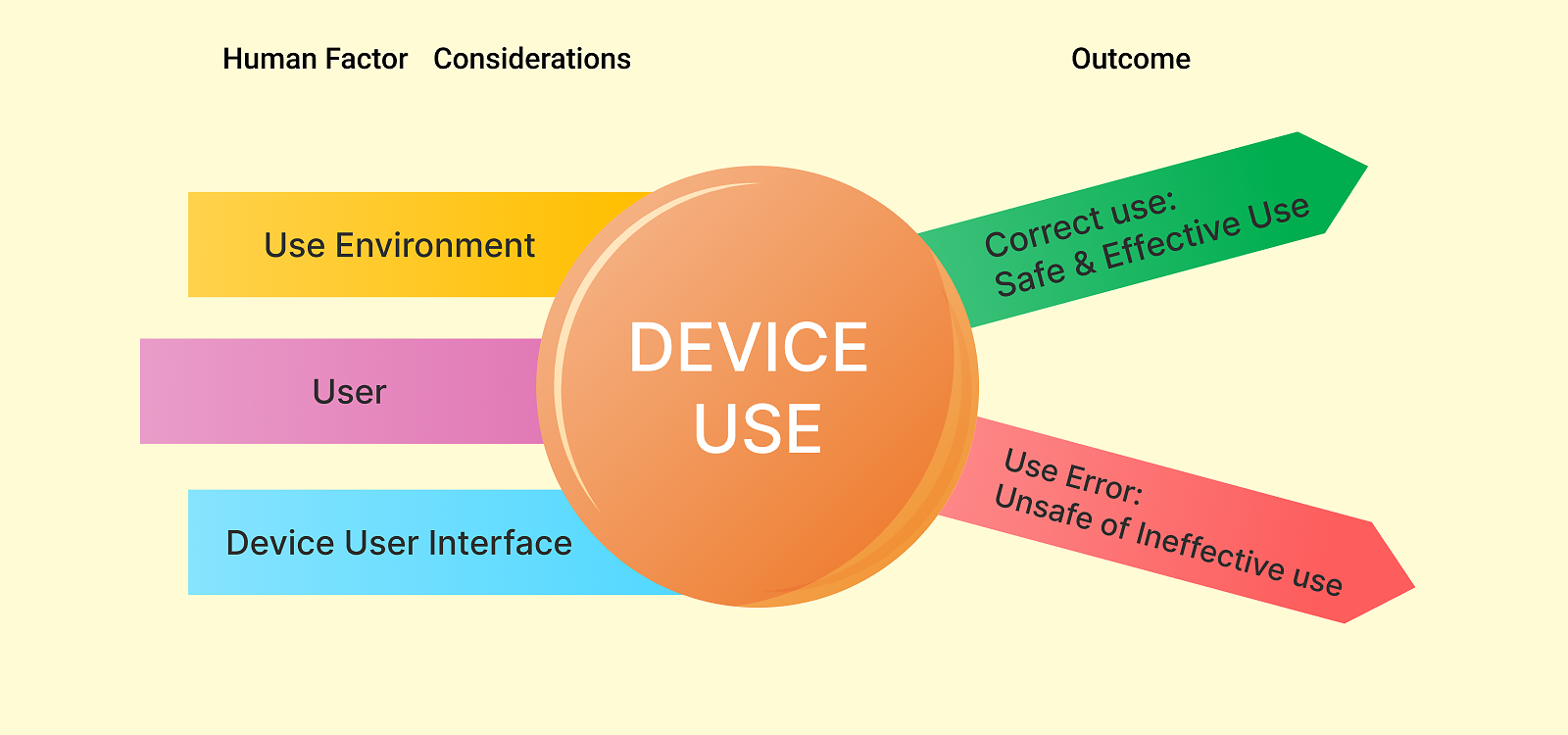
Human factors are used to design the user-device interface. The user interface includes all components that users interact with while preparing the device for use (e.g., unpacking, set up, and calibration), using the device, or performing maintenance (e.g., cleaning, replacing a battery and making repairs). Human factor considerations in the development of medical devices involve three major components of the device-user system:
- device users,
- device use environments, and
- device user interfaces.
The intended users of a medical device should be able to use the device without making errors that could compromise medical care or patient or user safety. The most important goal of the human factor process is to minimize use-related hazards and risks and confirm that these efforts were successful and that users can use the device safely and effectively. Compliance with usability requirements is mandatory for all medical devices.
[1] https://hub.jhu.edu/2016/05/03/medical-errors-third-leading-cause-of-death/