MedTech Europe Survey – Implementation of the MDR
MedTech Europe is the European trade association for the medical technology industry, including diagnostics, medical devices, and digital health. MedTech Europe's purpose is to make innovative medical technology available to more people while helping healthcare systems move towards a more sustainable path. In April 2022, the organization, in collaboration with the Medical Device Coordination Group (MDCG), surveyed a total of 475 companies (373 small and medium enterprises (SMEs) and 102 large companies) operating in the EU to analyze the availability of medical devices in 2022 in connection to the implementation of the new EU Medical Device Regulation (MDR). The survey represents an estimated 60-70% of market revenue coverage.
The responses by the companies that participated in the survey lead to the following conclusions:
- MDR certificates have not yet been issued for over 85% of the more than 500,000 devices previously certified under the old Medical Device Directive or the Active Implantable Medical Device Directive. According to the survey, since the first MDR certificates were issued in 2019, only 69,239 devices have been certified to the MDR in the past three years.
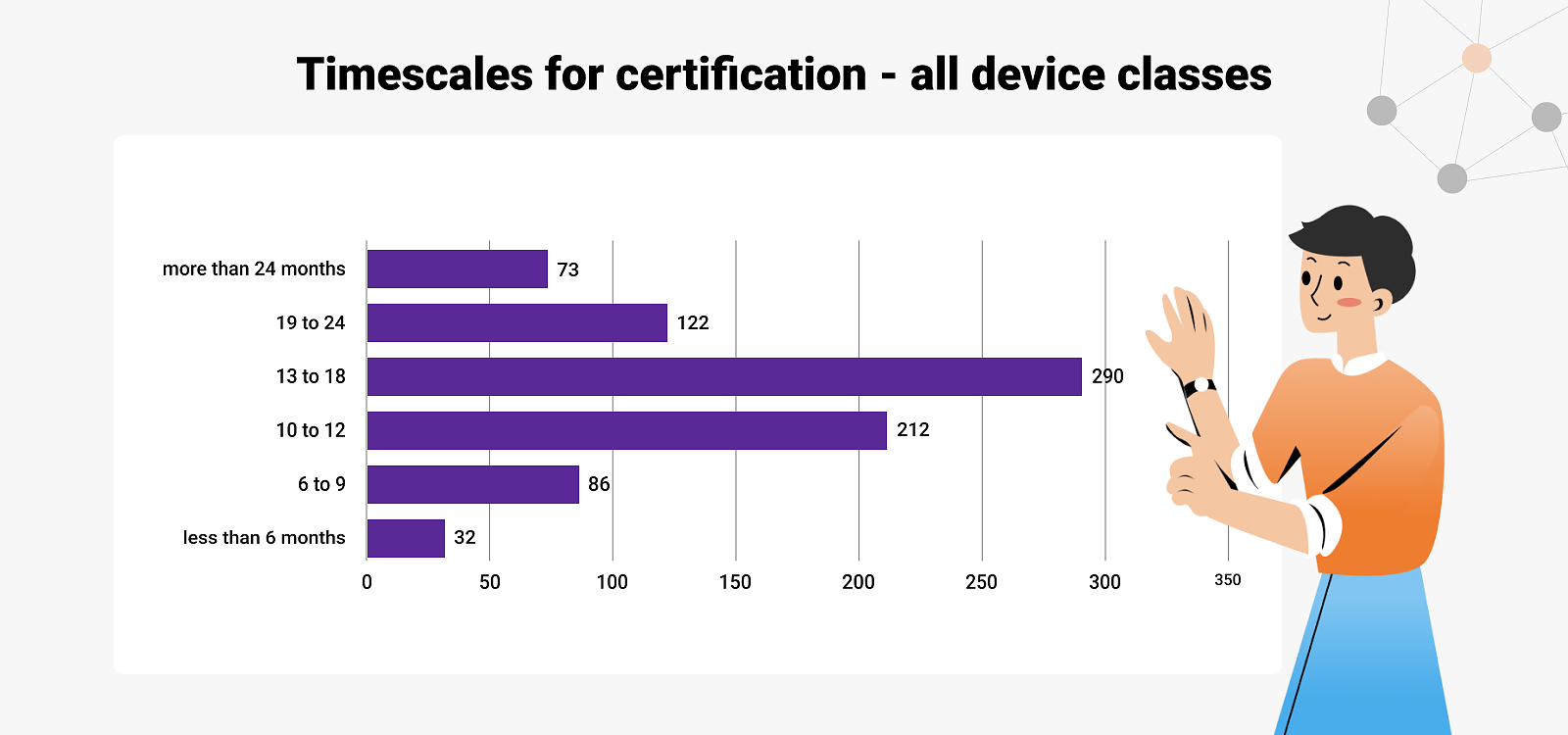
- The time-to-certification with MDR-designated Notified Bodies is slow: it takes 13-18 months on average across all devices and company sizes for initial certification. This is twice as long as it used to take for certification under the Directives.
- 54% of survey respondents said they do not intend to transition some of their portfolios to the MDR. All product categories are impacted by potential device discontinuations. This will have a significant impact on healthcare systems.
- SMEs face more challenges in the MDR implementation than larger companies. Up to 30% of SMEs have either no Notified Body (15%) and/or have a Notified Body that is not yet designated to the MDR (15%). For SMEs, progress to MDR certification is slower than average.
- The MDR has not supported innovation in the EU. Nearly half of the respondents are deprioritizing (or will do so) the EU market as the geography of choice for the first regulatory approval of new devices.
The conclusion of the survey report states: “The implementation of MDR in the EU is having a serious effect on the EU medical device market. This will be felt by EU patients and health systems. In addition to the disappearance of legacy devices, the survey data clearly shows that innovation is already leaving Europe. This too, MedTech Europe fervently believes, must also be course-corrected, otherwise, the clinical benefits of new and improved device designs will become preferentially available to patients in third countries first, requiring patients based in the EU to wait for the MDR system to become ready. This survey clearly indicates an urgent need for immediate action by decision-makers to help keep needed medical devices available in Europe.”
You can read the full survey report here.